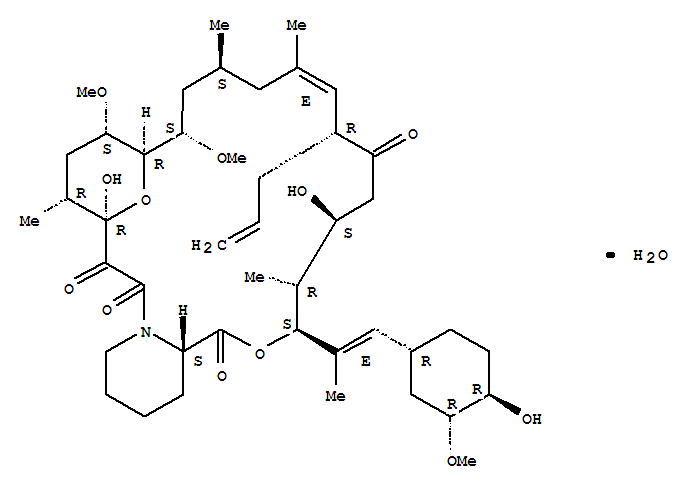
- Product Details
Keywords
- Offer Tacrolimus
- Tacrolimus with low price
- global trader Tacrolimus
Quick Details
- ProName: Global trader offer Tacrolimus 109581-...
- CasNo: 109581-93-3
- Molecular Formula: C44H69NO12.H2O
- Appearance: White or almost white crystalline powd...
- Application: 104987-11-3,109581-93-3,API
- DeliveryTime: promptly
- PackAge: As requirement
- Port: SHANGHAI
- ProductionCapacity: 5 Kilogram/Month
- Purity: 98.5%min
- Storage: As normal
- Transportation: AS NORMAL
- LimitNum: 1 Gram
- Related Substances: 1.5%Max
- Residue on Ignition: 0.2%Max
- Heavy Metal: 20PPM Max
- Valid Period: 2 years
- 109581-93-3: assay is 98%min
Superiority
Global trader offer Tacrolimus 109581-93-3 with low price
At the molecular level, the role of tacrolimus is obviously to combine with cellular protein (FKBP12) and accumulate in cells to produce effects. The FKBP12-tacrolimus complex specifically binds to and inhibits calcinurin, which inhibits the calcium ion-dependent signaling pathway produced in T cells, thereby preventing the transcription of discontinuous lymphokine genes. This drug is a highly immunosuppressive drug, and its activity has been confirmed in in vitro and in vivo experiments. This drug inhibits the formation of cytotoxic lymphocytes, which is the main role of transplant rejection. This drug inhibits the activation of T cells and the proliferation of T helper cells dependent on B cells. It also inhibits the production of lymphokines such as interleukin-2, interleukin-3 and gamma-interferon and the expression of interleukin-2 receptors. At the molecular level, the drug’s effect seems to be produced by binding to cellular protein (FKBP), which also causes the compound to accumulate in cells. In in vivo experiments, it was found that the drug has been shown to be effective for liver and kidney transplantation.
Details
Why work with us?
we go beyond the standard to produce high-quality, globally relevant pharmaceutical reference standards that you can trust. For more than 25 years we have delivered quality reference standards for analytical development, method validation, and stability and release testing to customers around the world.
Alongside a market-leading product range accredited to ISO 17034, the majority of our 5,000+ impurity, API and excipient reference standards are manufactured under ISO/IEC 17025 and accompanied by an extensive Certificate of Analysis.
With unparalleled depth of knowledge, decades of manufacturing experience and unrivalled scientific excellence, we work tirelessly to help you create ever better, safer medicines.

 Assessedsupplier
Assessedsupplier 

